
The progress followed a common pattern.įirst, a chemist identified a new lanthanide then a few years later, another scientist came along and extracted another lanthanide from the Because their properties are so similar, and because they are inclined to congregate in the same substances, the original isolation and identification of the lanthanides was an arduous task that took well over a century. If, on the other hand, rarity is understood not in terms of scarcity, but with regard to difficulty in obtaining an element in its pure form, then indeed the lanthanides are rare. The most plentiful of the lanthanides, cerium, has an abundance of 46 ppm, greater than that of tin. In terms of parts per million (ppm), thulium has a presence in Earth's crust equivalent to 0.2 ppm. As for rarity, the scarcest of the lanthanides, thulium, is more abundant than either arsenic or mercury, and certainly no one thinks of those as rare substances. Though they were once known as the rare earth metals, lanthanides were so termed because, as we shall see, they are difficult to extract from compounds containing other substances -including other lanthanides. Lanthanides tend to form ionic compounds, or compounds containing either positive or negative ions, with other substances -in particular, fluorine. This is the most stable ion for lanthanides, which sometimes develop less stable +2 or +4 ions. When a lanthanide reacts with another element to form a compound, it usually loses three of its outer electrons to form what are called tripositive ions, or atoms with an electric charge of +3. As noted earlier, they also are quite reactive with oxygen, and they experience combustion readily in air. Lanthanides react rapidly with hot water, or more slowly with cold water, to form hydrogen gas. Contamination also alters their boiling points, which range from 1,506.2 ☏ (819 ☌) for ytterbium to 3,025.4 ☏ (1,663 ☌) for lutetium. If contaminated with other metals, such as calcium, they corrode easily, and if contaminated with nonmetals, such as nitrogen or oxygen, they become brittle. Nonetheless, lanthanides tend to be rather "temperamental" as a class. The reactive tendencies of the other lanthanides vary: for instance, gadolinium and lutetium do not oxidize until they have been exposed to air for a very long time. Metal, these five lanthanides are kept stored in mineral oil. (An oxide is a compound formed by metal with an oxygen.) To prevent this result, which tarnishes the When exposed to oxygen, they form an oxide coating. Lanthanum, cerium, praseodymium, neodymium, and europium are highly reactive. PROPERTIES OF LANTHANIDES.īright and silvery in appearance, many of the lanthanides -though they are metals -are so soft they can be cut with a knife. Most of these are discussed individually in this essay.
#Periodic table series series
As their name indicates, members of the lanthanide series share certain characteristics with lanthanum hence the collective term "lanthanides." These 15 elements, along with their chemical symbols, are: The lanthanide series is usually combined with lanthanum, which has an atomic number of 57, under the general heading of lanthanides. The latter appear in groups 3 through 12 on the IUPAC version of the periodic table, though they are not numbered on the North American version. The lanthanides and actinides are actually "branches" of the larger family known as transition metals. Specifically, the lanthanides and actinides are the only elements that fill the f-orbitals. These 14, along with the actinides -atomic numbers 90 through 103 -are set aside from the periodic table due to similarities in properties that define each group. The lanthanide series consists of the 14 elements, with atomic numbers 58 through 71, that follow lanthanum on the periodic table of elements.
#Periodic table series tv
Though some lanthanides have limited uses, members of this group are found in everything from cigarette lighters to TV screens, and from colored glass to control rods in nuclear reactors. They are, however, difficult to extract, a characteristic that defines them as much as their silvery color sometimes high levels of reactivity and sensitivity to contamination.
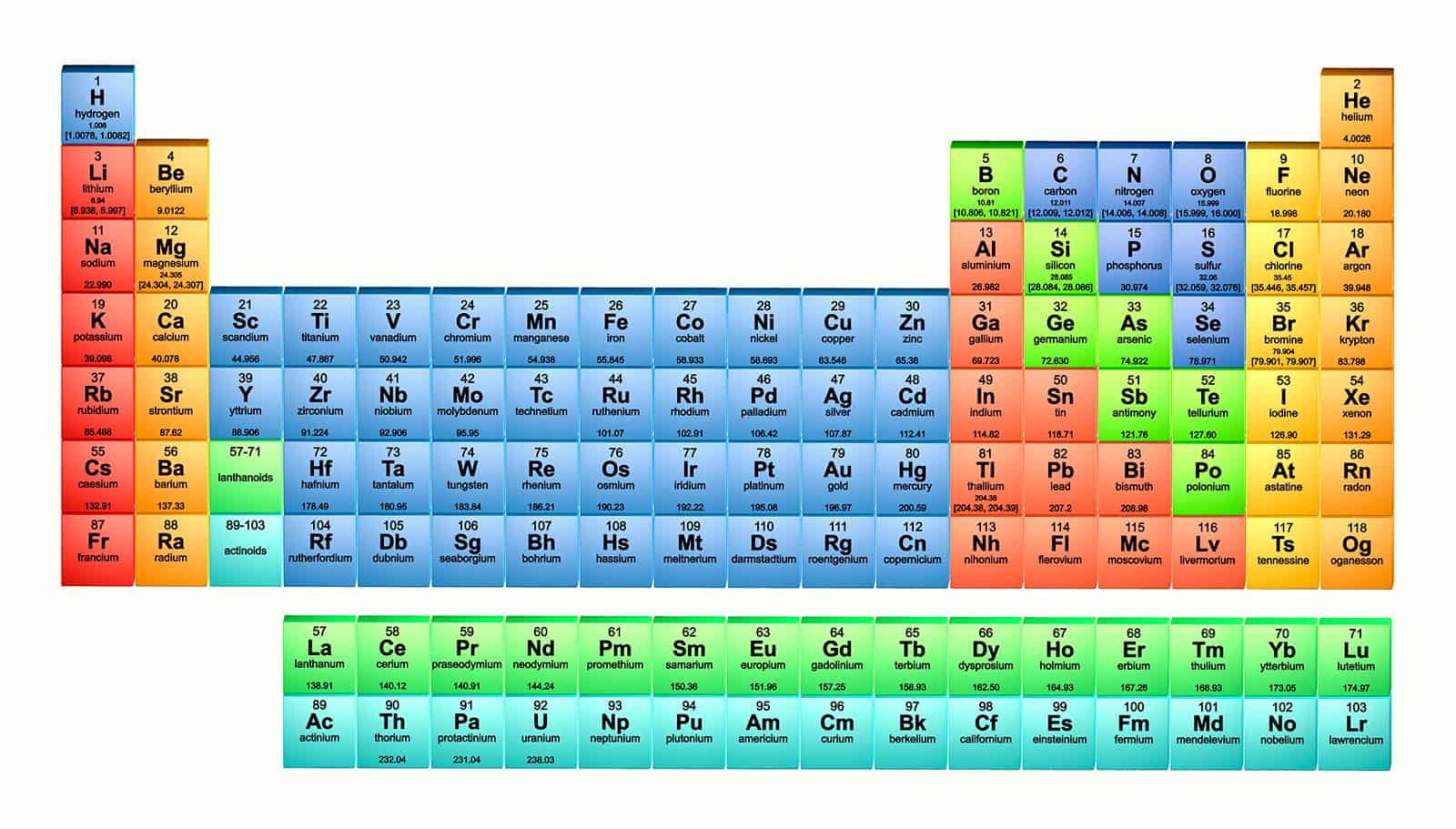
In fact, they are not particularly rare: many of them appear in as much abundance as more familiar elements such as mercury. Composed of lanthanum and the 14 elements of the lanthanide series, the lanthanides were once called the "rare earth" metals. Along the bottom of the periodic table of elements, separated from the main body of the chart, are two rows, the first of which represents the lanthanides.


 0 kommentar(er)
0 kommentar(er)
